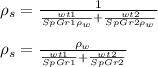
Answer:
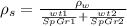
The only other data you need is the density of water ρw.
Step-by-step explanation:
We can start by the volume balance
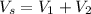
We can replace the volumes with V=M/ρ
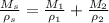
If we divide every term by Ms
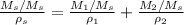
By definition, wt=Mi/Msol, so we can replace that in the expression
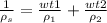
Then we have the expression of the density of the solution
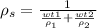
To replace ρ1 and ρ2, you have to multiply the specific gravity of the components and the density of water.