Answer:
The molar composition of the equilibrium mixture is
NO: 0.338 = 33.8%
Br2: 0.169 = 16.9%
NOBr: 0.493 = 49.3%
Step-by-step explanation:
The reaction of nitrosyl bromide formation can be written as
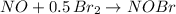
To form 1 mol of NOBr, we need 1 mol of NO and 0.5 mol of Br2.
A sample of 0.0524 mol NO with 0.0262 mol Br2 gives an equilibrium mixture containing 0.0311 mol NOBr.
Then, to form 0.0311 mol NOBr, were needed 0.0311 mol NO and 0.01555 mol of Br2.
The amount of NO that stay in the same form is (0.0524-0.0311)=0.0213 mol NO.
The amount of Br2 that stay in the same form is (0.0262-0.01555)=0.01065 mol Br2.
The total amount of mol is (0.0213 mol NO + 0.01065 mol Br2 + 0.0311 mol NOBr) = 0.06305.
The molar composition is
NO: 0.0213/0.06305 = 0.338 = 33.8%
Br2: 0.01065/0.06305 = 0.169 = 16.9%
NOBr: 0.0311/0.06305 = 0.493 = 49.3%