Step-by-step explanation:
The given data is as follows.
 = 211 nm,
= 211 nm,
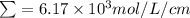
l = 1 cm, 7% < Transmittance < 85%
Suppose the aqueous solution follows Lambert-Beer's law. Therefore,
Absorbance =
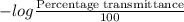
Hence, for 7% transmittance the value of absorbance will be as follows.
Absorbance =
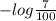
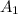 = 1.155
= 1.155
For 85% transmittance the value of absorbance will be as follows.
Absorbance =
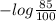
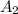 = 0.07058
= 0.07058
According to Lambert-Beer's law.
A =
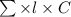
where, A = absorbance
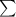 = molar extinction coefficient
= molar extinction coefficient
C = concentration
Therefore, concentration for 7% absorbance is as follows.
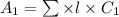
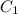 =
=
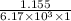
=
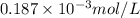
= 0.187 mmol/L
Concentration for 85% absorbance is as follows.
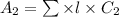
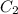 =
=
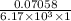
=
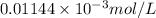
= 0.01144 mmol/L
Thus, we can conclude that linear range of phenol concentration is 0.01144 mmol/L to 0.187 mmol/L.