Answer:
Final temperature is 302 K
Step-by-step explanation:
You can now initial volume with ideal gas law, thus:
V =
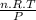
Where:
n are moles: 2 moles
R is gas constant: 0,082
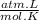
T is temperature: 300 K
P is pressure: 1 atm
V is volume, with these values: 49,2 L
The work in the expansion of the gas, W, is: 1216 J - 34166 J = -32950 J
This work is:
W = P (Vf- Vi)
Where P is constant pressure, 1 atm
And Vf and Vi are final and initial volume in the expansion
-32950 J = -1 atm (Vf-49,2L) ×
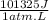
Solving: Vf = 49,52 L
Thus, final temperature could be obtained from ideal gas law, again:
T =
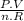
Where:
n are moles: 2 moles
R is gas constant: 0,082
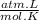
P is pressure: 1 atm
V is volume: 49,52 L
T is final temperature: 302 K
I hope it helps!