Answer:
a) 44.442 grams of aluminium chloride is produced.
b) 8.996 grams of the excess reactant that id aluminum is left unreacted.
Step-by-step explanation:
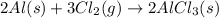
Moles of aluminum =
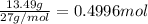
Moles of chlorine gas =
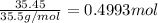
According to reaction, 1 mol aluminum reacts with 3 moles of chlorine gas.
Then 0.4996 moles of aluminum will react with:
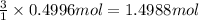 of chlorine gas
of chlorine gas
Then 0.4993 moles of chlorine gas will react with:
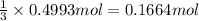 of aluminum
of aluminum
As we can see that moles of an aluminium are in excess. Hence, aluminium is an excess reagent.
So moles of aluminum chloride will depend upon moles of chlorine gas.
According to reaction 3 moles of chlorine gas give 2 moles of aluminium chloride.
Then 0.4993 mole of chlorine gas will give:
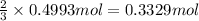 of aluminium chloride .
of aluminium chloride .
Mass of 0.3329 moles of aluminium chloride :
=0.3329 mol × 133.5 g/mol= 44.442 g
44.442 grams of aluminium chloride is produced.
Moles of aluminium left unreacted = 0.4996 mol - 0.1664 mol = 0.3332 mol
Mass of 0.3332 moles of aluminum :
0.3332 mol × 27 g/mol = 8.996 g[/tex]
8.996 grams of the excess reactant that id aluminum is left unreacted.