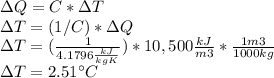Answer:
(a) 140 F
(b) The temperature rise at the point where the heat is dumped is 2.51 degC
Step-by-step explanation:
(a) Considering T1 the temperature of input of the first engine, T2 the temperature of the exhaust of the first engine (and input of the second engine) and T3 the exhaust of the second engine, if both engines have the same efficiency we have:
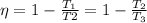
The temperatures have to be expressed in Rankine (or Kelvin) degrees
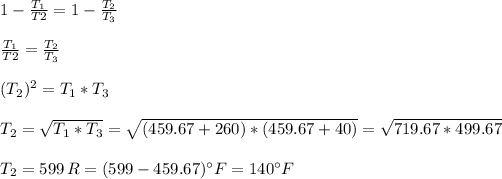
(b) The Carnot efficiency of the cycle is
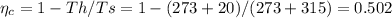
If the efficiency of the plant is 60% of the Carnot efficiency, we have
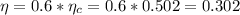
The heat used in the plant can be calculated as
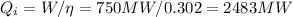
And the heat removed to the heat sink is
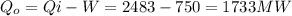
If the flow of the river is 165 m3/s, the heat per volume in the sink is
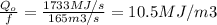
Considering a heat capacity of water C=4.1796 kJ/(kg*K) and a density ρ of 1000 kg/m3, the temperature rise of the water is