Answer:
There are 550.5 moles of methanol in 110.0 kilograms of the mixture.
Step-by-step explanation:
A solution 15% weight of methanol means there is 15g of methanol per 100g of the mixture or 0.1kg of the mixture. Also, the molar mass of methanol (CH3OH) is:
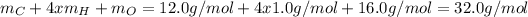
Thus, dividing 15g by molar mass
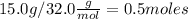 we find there is 0.5 moles of methanol per 0.1Kg of the mixture. Calculating the number of mols of methanol in 110.0 kilograms of the mixture:
we find there is 0.5 moles of methanol per 0.1Kg of the mixture. Calculating the number of mols of methanol in 110.0 kilograms of the mixture:
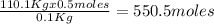
Therefore, there is 550.5 moles of methanol in 110.0 kilograms of the mixture.