Answer : The overall mixture flow rate is 11905.71 lb.mass/hour
Explanation :
As we are given that 16.0 weight percent methanol. That means, 16.0 g of methanol present in 100 g of mixture (methanol-methyl acetate).
Mass of methanol = 16.0 g
Mass of mixture = 100 g
Mass of methyl acetate = 100 - 16.0 = 84.0 g
Now converting flow rate of methyl acetate from lb.mols/hour to lb.mass/hour.
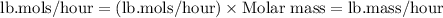
Molar mass of methyl acetate = 74.08 g/mols
So,
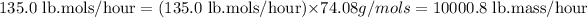
Now we have to calculate the overall mixture flow rate.
As, 84 g of methyl acetate flow rate = 10000.8 lb.mass/hour
So, 100 g of mixture flow rate =
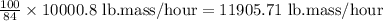
Therefore, the overall mixture flow rate is 11905.71 lb.mass/hour