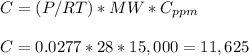Answer:
(a) 17,178 mg/m3
(b) 11,625 mg/m3
Step-by-step explanation:
The concentration of CO in mg/m3 can be calculated as
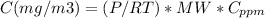
For standard conditions (1 atm and 25°C), P/RT is 0.0409.
Concentration of 1.5% percent by volume of CO is equivalent to 1.5*10,000 ppm= 15,000 ppm CO.
The molecular weigth of CO is 28 g/mol.
(1) For 25°C and 1 atm conditions
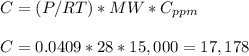
(b) For 200°C and 1.1 atm,
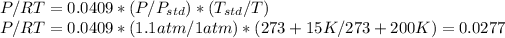
Then the concentration in mg/m3 is