Answer:
The volume is 6375 L.
Step-by-step explanation:
The reaction is carried out in liquid phase and it is second order reaction.
Reaction rate must be like this
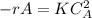
Constant reaction rate is K = 0.04
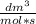 .
.
As 1
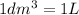 we can work indistinctly with Liters or cubic decimeters.
we can work indistinctly with Liters or cubic decimeters.
Inlet concentration is CAo = 1.9 M. Outlet concentration is CAf = 0.2 M.
We can suppose homogeneous and uniform conditions inside the reactor, the same of the outlet. So, concentration inside the reactor should be equal to CAf.
Volumetric flow is vo constant at
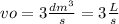 .
.
Design equation for CSTR reactor is showed below. Where V is the reactor volume, FAo is mass flow inlet of reactant, FAf is outle flow of reactant:
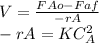
We can calculate mass flow of reactant at the input and output using the volumetric flow of the system. As it is shown:
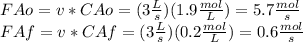
Finally we can replace in the design equation. Where CA in the reaction rate should be the same value of the output due to the assumption of perfect mixing inside the CSTR.
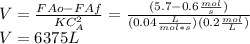
Finally, the volume is 6375 L for the CSTR previously showed.