Step-by-step explanation:
It is known that for
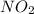 , ppm present in 1
, ppm present in 1
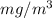 are as follows.
are as follows.
1
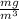 = 0.494 ppm
= 0.494 ppm
So, 150
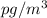 =
=
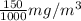
= 0.15
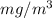
Therefore, calculate the equivalent concentration in ppm as follows.
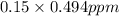
= 0.074 ppm
Thus, we can conclude that the equivalent concentration in ppm at STP is 0.074 ppm.