Answer:
No
Step-by-step explanation:
The mass fraction is defined as:
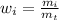
where:
- wi: mass fraction of the substance i
- mi: mass of the substance i
- mt: total mass of the system
The mass fraction of two substances (A and B), will be the same, ONLY if the mass of the substance A (mA) is the same as the mass of the substance B (mB).
An equimolar mixutre of O2 and N2 has the same amount of moles of oxygen and nitrogen, just to give an example let's say that the system has 1 mole of O2 and 1 mole of N2. Then using the molecuar weigth of each of them we can calculate the mass:
mA= 1 mole of O2 * 16 g/1mol = 16 g
mB=1 mole of N2 *28 g/1mol=28 g
As mA≠mB then the mass fractions are not equal, so the answear is NO.