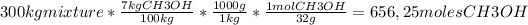Answer:
656,25 moles of CH3OH
Step-by-step explanation:
If the mixture has 7% of weight in methanol (CH3OH), it means that for every 100 kg of the mixture there are 7 kg of methanol.
To solve the problem we just use this relation and convert the kilograms of methanol to gmoles of methanol using the molecuar weight of the methanol (32g/mol):