Answer:
The reaction rate k is 0.0012563 (1/hour).
Step-by-step explanation:
We considered the reactions occurring in the plant as first order, and represented by this equation:
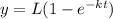
where y is the BOD at time t, L is the initial value of BOD and k is the reaction rate.
If we replaced with the values
y = 2 mg O2/l (1% of the initial value)
L = 200 mg 02/l
t = 8 hr
We can calculate k
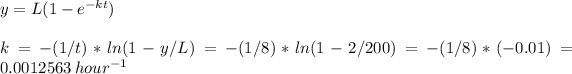
The reaction rate k is 0.0012563 1/hour.