Answer: 26138g/mol
Step-by-step explanation:

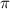 = osmotic pressure = 12 mmHg =
= osmotic pressure = 12 mmHg =
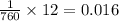 atm (760 mmHg= 1atm)
atm (760 mmHg= 1atm)
C= concentration in Molarity
R= solution constant = 0.0821 Latm/Kmol
T= temperature =
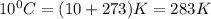
For the given solution: 18 g of macromolecule is dissolved to make 1 L of solution.
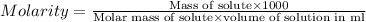
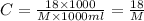
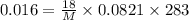
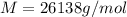
The molecular weight of the macromolecule in grams per mole is 26138.