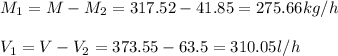Answer:
Volumetric flow:
Mixture = 373.55 l/h; Benzene = 310.05 l/h; n-hexane = 63.5 l/h
Mass flow
Mixture = 317.52 kg/h; Benzene = 275.66 kg/h; n-hexane = 41.85 kg/h
Step-by-step explanation:
First, we can express the mixture mass flow as
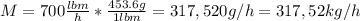
The volumetric flow of the mixture is
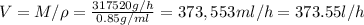
The mass balance can be written as
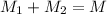
And the volumetric flow as
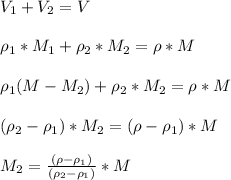
If fluid 2 is n-hexane, we have
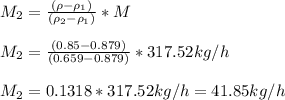
The mass flow of n-hexane is 41.85 kg/h.
Its volumetric flow is 63.5 litre/h
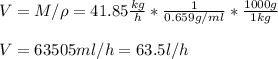
The mass flow of benzene can be deducted by difference: