Answer:
Force of attraction = 35.96
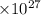 N
N
Step-by-step explanation:
Given: charge on anion = -2
Charge on cation = +2
Distance = 1 nm =
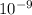 m
m
To calculate: Force of attraction.
Solution: The force of attraction is calculated by using equation,
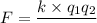 ---(1)
---(1)
where, q represents the charge and the subscripts 1 and 2 represents cation and anion.
k =
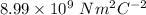
F = force of attraction
r = distance between ions.
Substituting all the values in the equation (1) the equation becomes
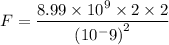
Force of attraction = 35.96
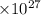 N
N