Answer: Option (C) is the correct answer.
Step-by-step explanation:
It is given that total mass is 1 kg and quality is 50%. Hence, the weight of liquid and vapor is 0.5 kg each.
Since, temperature is given as
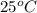 = (25 + 273.15) K = 298.15 K
= (25 + 273.15) K = 298.15 K
Molecular mass of water = 18 g/mol
Therefore, calculate the number of moles of water as follows.
No. of moles of water vapor =

=
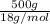 (as 1 kg = 1000 g, so 0.5 kg =
(as 1 kg = 1000 g, so 0.5 kg =
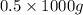 = 500 g)
= 500 g)
= 27.78 mol
This will also be equal to the number of moles of liquid water present.
According to the steam tables, water exists in its saturated state at
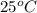 at a pressure
at a pressure
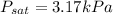 or 3170 Pa.
or 3170 Pa.
Hence, on assuming ideal gas behavior of the vapor the equation will be as follows.
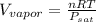
=
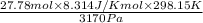
= 21.72

Whereas we will calculate the volume of liquid water as follows.
Volume =
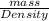
=
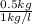
= 0.5 L
As 1 L = 0.001
 . So, 0.5 L = 0.0005
. So, 0.5 L = 0.0005
 .
.
Therefore, total volume = Volume of vapor + volume of liquid water
= 21.72
 + 0.0005
+ 0.0005

= 21.7205

= 22
 (approx)
(approx)
Thus, we can conclude that volume occupied by the water (vapor liquid) is 22
 .
.