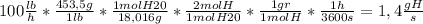Answer:
i) 0,7 molH20/s
ii)11,2 g O/s
iii)1,4 g H/s
Step-by-step explanation:
i) To find the molar flow rate of water, we just convert the mass of water to moles of water using its molecular weight(g/mol) and changing to the proper units (lb to grames and hours to seconds):

ii) Now we just consider the oxygen in the water stream (for 1 mole of water there is 1 mole of oxygen):
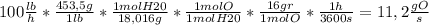
iii)Just considering the hydrogen in the stream (for 1 mole of water there is 2 moles of hydrogen):