Answer: The fraction of the bonding that is ionic is 0.08875.
Step-by-step explanation:
Percentage ionic character is calculated using the formula:
% ionic character =
![[16* \Delta E_N + 3.5* (\Delta E_N)^2]](https://img.qammunity.org/2020/formulas/chemistry/college/ggu615xxudqz3n8darn16ns42tjrpq33zm.png)
Where,
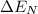 = electronegativity difference
= electronegativity difference
Given : The electronegativities for Ga and P are 1.6 and 2.1 respectively.
Thus
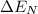 = electronegativity of phosphorous - electronegativity of galium = 2.1 -1.6 = 0.5
= electronegativity of phosphorous - electronegativity of galium = 2.1 -1.6 = 0.5
Therefore,
%ionic character =
![[16* 0.5 + 3.5* (0.5)^2]=8.875](https://img.qammunity.org/2020/formulas/chemistry/college/b5979rezjypsp6lei883h11bth3tzz3q8f.png)
Fraction of ionic bond =
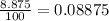
The fraction of the bonding that is ionic is 0.08875.