Answer:
The answers is the option a: 46.062
Step-by-step explanation:
First, you must determine the limiting reagent, that is the reagent that is consumed first during the reaction.
So, in first place, you must determine the molecular weight of the molecules CH4, O2, CO2 and H20. You know that
- C: 12 kg/kmol
- H: 1 kg/kmol
- 0: 16 kg/kmol
So, to determine the mass of a molecule, you must multiply the individual masses of each atom by the amount present in the molecule. This would be:
- CH4: 12 kg/kmol + 4* 1 kg/kmol= 16 kg/kmol because you have 1 C and 4 H in the CH4.
In the same way, you can determinate the mass of all reagents and products involved in the reaction.
- O2: 2*16 kg/kmol=32 kg/kmol
- CO2: 12 kg/kmol+2*16 kg/kmol= 44 kg/kmol
- H2O: 2*1 kg/kmol + 16 kg/kmol= 18 kg/kmol
Now you can apply stoichiometry to determine the limiting reagent using these numbers and observing how many molecules react.
On one side, it is known that, by stoichiometry, 1 mol of CH4 and 2 moles of O2 react. This means that 16 kg/mol of CH4 and 64 kg/kmol ( 2moles* 32 kg/kmol) of O2 react
And it is known that there are 71 kg/hr of CH4 reacting with 67 kg/hr of 02
So, using the stoichiometric information (16 kg/mol of CH4 and 64 kg/kmol of O2), 71 kg/hr of CH4 and The Rule of Three, you can determine the limiting reagent:
16 kg/kmol CH4 ⇒ 64 kg/kmol O2 (stoichiometry)
71 kg/kmol CH4 ⇒ x
So
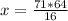
x=284 kg/kmol
This means that to react 71 kg/kmol of CH4, 284 kg/kmol of O2 are needed. But you only have 67 kg/mol that can react. That is why O2 is the limiting reagent, because it is consumed first.
Now, you can calculate the rate of CO2 that is generated, using the data of the amount of limiting reagent and stoichiometry. This is:
64 kg/kmol O2 ⇒ 44 kg/kmol CO2 (stoichiometry)
67 kg/kmol O2 ⇒ x
So
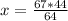
x=46.0625 kg/kmol
This means that 46.0625 kg/kmol of CO2 are generated.