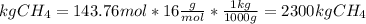Answer:
2300 kg of methane will need to be combusted.
Step-by-step explanation:
The enthalpy of this combustion is -882 kJ/mol CH4.
To generate 126799 kJ of energy, we need
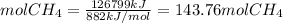
The molecular weight of CH4 is (12+4*1)=16 g/mol
Then, the mass needed to generate 126799 kJ of energy is