Answer:
2,0894x10¹² tonnes of CO₂ in atmosphere
Step-by-step explanation:
The total mean mass of the atmosphere is 5.1480×10¹⁸ kg. CO₂ is an important race gas in Earth's atmosphere because it is part of the carbon cycle.
405 ppm of CO₂ in atmosphere means 405 mg of CO₂ per kg of atmosphere. If the mass of the atmosphere is 5.1480×10¹⁸ kg:
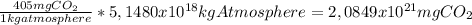
In tonnes:
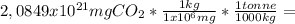 2,0894x10¹² tonnes of CO₂ in atmosphere
2,0894x10¹² tonnes of CO₂ in atmosphere
I hope it helps!