Answer:
The required volume of hexane is 0.66245 Liters.
Step-by-step explanation:
Volume of octane = v=1.0 L=
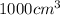
Density of octane= d =
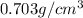
Mass of octane ,m=
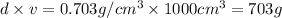
Moles of octane =
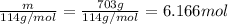
Mole percentage of Hexane = 45%
Mole percentage of octane = 100% - 45% = 55%
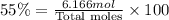
Total moles = 11.212 mol
Moles of hexane :
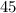
Moles of hexane = 5.0454 mol
Mass of 5.0454 moles of hexane,M = 5.0454 mol × 86 g/mol=433.9044 g
Density of the hexane,D =
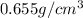
Volume of hexane = V
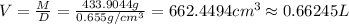
(1 cm^3= 0.001 L)
The required volume of hexane is 0.66245 Liters.