Answer:
a) 63,0%
b) 54,4 Pounds HNO₃ per cubic foot of solution
c) 13,8 M
Step-by-step explanation:
a) Weight percent is the ratio solute:solution times 100:
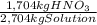 = 63,0%
= 63,0%
b) Pounds HNO₃ per cubic foot of solution at 20 °c
Pounds HNO₃:
1,704 kg
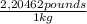 = 3,7567 pounds
= 3,7567 pounds
Cubic foot:
2,704 kg
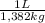 x
x
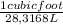 = 0,069 ft³
= 0,069 ft³
Thus:
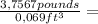 = 54,4 Pounds HNO₃ per cubic foot of solution
= 54,4 Pounds HNO₃ per cubic foot of solution
c) Moles of HNO₃:
1704 g HNO₃
 = 27,04 moles
= 27,04 moles
Liters of solution:
2,704 kg
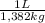 = 1,96 L of solution
= 1,96 L of solution
Molarity:
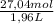 = 13,8 M
= 13,8 M