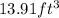Answer: The volume of given amount of argon is
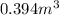 and
and
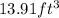
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of argon = 3.5 kg = 3500 g (Conversion factor: 1 kg = 1000 g)
Molar mass of argon = 40 g/mol
Putting values in above equation, we get:
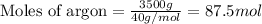
To calculate the volume of gas, we use the equation given by ideal gas equation:

where,
P = pressure of the gas = 550 kPa
V = Volume of gas = ?
n = number of moles of argon = 87.5 moles
R = Gas constant =
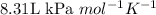
T = temperature of the gas =
![25^oC=[25+273]=298K](https://img.qammunity.org/2020/formulas/chemistry/college/wtxcecdloxvsvyk6txukrf7xqizrrmrtjc.png)
Putting values in above equation, we get:
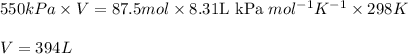
Converting volume from liters to cubic meters and cubic foot, we use the conversion factor:
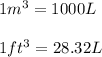
Converting the given volume into cubic meters:
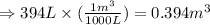
Converting the given volume into cubic foot:
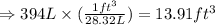
Hence, the volume of given amount of argon is
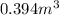 and
and