Step-by-step explanation:
According to Clausius-Claperyon equation,
![ln ((P_(2))/(P_(1))) = \frac{-\text{heat of vaporization}}{R} * [(1)/(T_(2)) - (1)/(T_(1))]](https://img.qammunity.org/2020/formulas/chemistry/college/4q95k6oc65w2h9io1by8cp7xpjxqi0tlwg.png)
The given data is as follows.
 = (63.5 + 273) K
= (63.5 + 273) K
= 336.6 K
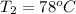 = (78 + 273) K
= (78 + 273) K
= 351 K
 = 1 atm,
= 1 atm,
 = ?
= ?
Putting the given values into the above equation as follows.
![ln ((P_(2))/(P_(1))) = \frac{-\text{heat of vaporization}}{R} * [(1)/(T_(2)) - (1)/(T_(1))]](https://img.qammunity.org/2020/formulas/chemistry/college/4q95k6oc65w2h9io1by8cp7xpjxqi0tlwg.png)
![ln ((1.75 atm)/(1 atm)) = \frac{-\text{heat of vaporization}}{8.314 J/mol K} * [(1)/(351 K) - (1)/(336.6 K)]](https://img.qammunity.org/2020/formulas/chemistry/college/dn4inzn89n2r53gz0j47q6dhr93f1g9bn9.png)
 =
=
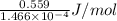
=
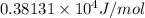
= 3813.1 J/mol
Thus, we can conclude that the heat of vaporization of ethanol is 3813.1 J/mol.