Step-by-step explanation:
The given data is as follows.
Mass of apple sauce mixture = 454 kg
Heat added (Q) = 121300 kJ
Heat capacity (
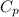 ) of apple sauce at
) of apple sauce at
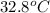 = 4.0177
= 4.0177
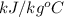
So, Heat given by heat exchanger = heat taken by apple sauce
Q =
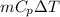
or, Q =
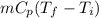
Putting the given values into the above formula as follows.
Q =
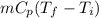
121300 kJ =
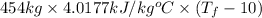
 =
=
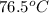
Thus, we can conclude that outlet temperature of the apple sauce is
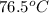 .
.