Answer:
751.8kg/m3
Explanation:
Assuming water's density is 1,000 kg/m3 and acetone's density is 791kg/m3
we use the formula
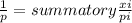
where 1/p is the density of the mixture, xi is the mass fraction and pi is the individual density of the components of the mixture.
To calculate xi we consider that acetone and water are in a 50% proportion, this means xi is 0.5 for both substances, now we can substitute in the formula:
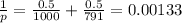
now we obtain p.
p=751.8kg/m3
I hope you find this information useful! good luck!