Answer:
a) Molar fraction:
Methane: 67,5%
Ethane: 25,1%
Propane: 7,4%
b) Molecular weight of the mixture: 25,16 g/mol
Step-by-step explanation:
with a basis of 100 kg:
Moles of methane:
500 g ×
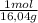 = 31,2 moles
= 31,2 moles
Mass of ethane
350 g ×
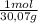 = 11,6 moles
= 11,6 moles
Mass of propane
150 g ×
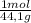 = 3,4 moles
= 3,4 moles
Total moles: 31,2 moles + 11,6 moles + 3,4 moles = 46,2 moles
Molar fraction of n-methane:
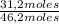 =67,5%
=67,5%
Molar fraction of ethane:
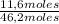 = 25,1%
= 25,1%
Molar fraction of propane:
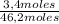 = 7,4%
= 7,4%
b) Average molecular weight:
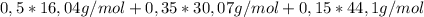 = 25,16g/mol
= 25,16g/mol
I hope it helps!