Answer:
3,48 mL of 8,00wt% H₂SO₄
Step-by-step explanation:
The equilibrium in water is:
H₂O (l) ⇄ H⁺ (aq) + OH⁻ (aq)
The initial concentration of [H⁺] is 10⁻⁸ M and final desired concentration is [H⁺] =
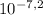
Thus, you need to add:
[H⁺] =
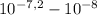 = 5,31x10⁻⁸ M
= 5,31x10⁻⁸ M
The total volume of the pool is:
5,00 m × 15,0 m ×1,50 m = 112,5 m³ ≡ 112500 L
Thus, moles of H⁺ you need to add are:
5,31x10⁻⁸ M × 112500 L = 5,97x10⁻³ moles of H⁺
These moles comes from
H₂SO₄ → 2H⁺ +SO₄²⁻
Thus:
5,97x10⁻³ moles of H⁺ ×
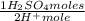 = 2,99x10⁻³ moles of H₂SO₄
= 2,99x10⁻³ moles of H₂SO₄
These moles comes from:
2,99x10⁻³ moles of H₂SO₄ ×
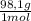 ×
×
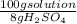 ×
×
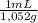 = 3,48 mL of 8,00wt% H₂SO₄
= 3,48 mL of 8,00wt% H₂SO₄
I hope it helps!