Answer:
Mass fraction: 73,6% n-hexane; 26,4% dichloromethane
Mole fraction: 73,0% n-hexane; 27,0% dichloromethane
Step-by-step explanation:
With a basis of 100 mL:
Mass of n-hexane:
85 mL ×
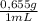 = 55,7 g
= 55,7 g
Mass of dichloromethane
15 mL ×
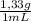 = 20,0 g
= 20,0 g
Total mass = 20,0 g + 55,7 g = 75,7 g
Mass fraction of n-hexane:
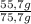 =73,6%
=73,6%
Mass fraction of dichloromethane:
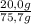 = 26,4%
= 26,4%
Moles of n-hexane:
55,7 g ×
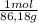 = 0,65 moles
= 0,65 moles
Mass of dichloromethane
20,0g ×
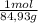 = 0,24 moles
= 0,24 moles
Total moles: 0,65 moles + 0,24 moles = 0,89 moles
Molar fraction of n-hexane:
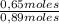 =73,0%
=73,0%
Molar fraction of dichloromethane:
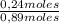 = 27,0%
= 27,0%
I hope it helps!