Answer:
A. 10.25
Step-by-step explanation:
Pkb =4.77
So pka = 14 - pka = 9.23
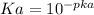
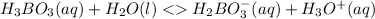
Initial 0.50M 0 0
Change -x +x +x
Equilibrium 0.50M-x +x +x
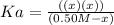
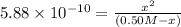
(-x is neglected) so we get
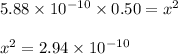
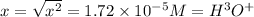
![pH=-log[H^3 O^+]\\\\pH=-log[1.72*10^(-5)]\\\\pH=4.76](https://img.qammunity.org/2020/formulas/chemistry/middle-school/5qhvo23qf5bizoy2q4u4fa2isepzhxxwxg.png)
pOH = 14 - pH
= 14 - 4.76
pOH = 9.24 is the answer
Option A - 10.25 is the answer which is close to 9.24