Answer: It will take 6.93 sec for tracer concentration to drop by 50% and 13.9 sec for tracer concentration to drop by 75%
Step-by-step explanation:
Expression for rate law for first order kinetics is given by:
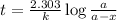
where,
k = rate constant =
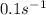
t = age of sample
a = let initial amount of the reactant = 100 g
a - x = amount left after decay process
a) for tracer concentration to drop by 50%
a - x = amount left after decay process = 50
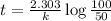
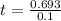
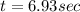
It will take 6.93 sec for tracer concentration to drop by 50%
b) for tracer concentration to drop by 75 %
a - x = amount left after decay process = 25
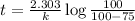
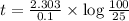
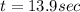
It will take 13.9 sec for tracer concentration to drop by 75%.