Answer:
 of arginine is higher than that of lysine.
of arginine is higher than that of lysine.
Step-by-step explanation:
1. According to the Bronsted-Lowry acid-base theory, a stronger base will have a weak conjugate acid.
The positive charge is more stable on
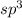 hybrid atom as compared to
hybrid atom as compared to
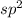 hybrid atom.
hybrid atom.
Therefore, arginine is more basic as compared to lysine.
2. Moreover, 3 nitrogen atoms are present in arginine that has the capability to accept acidic protons as compared to the one nitrogen that is present in lysine.
Higher the
 , higher will be the basicity.
, higher will be the basicity.
Therefore,
 of arginine is higher than that of lysine.
of arginine is higher than that of lysine.