Answer:
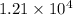 cal would be needed to heat 5.0 lbs of copper from 22 degrees C to 80.0 degrees C.
cal would be needed to heat 5.0 lbs of copper from 22 degrees C to 80.0 degrees C.
Step-by-step explanation:
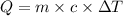
where
 = Final T - Initial T
= Final T - Initial T
Q is the heat energy in calories
c is the specific heat capacity (for copper 0.092 cal/(g℃))
m is the mass of water
plugging in the values
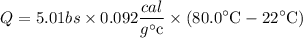
Please Note:
1 lb = 453.592grams
So,
5 lbs = 5 × 453.592g = 2268 g
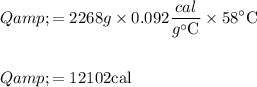
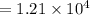 cal (Answer)
cal (Answer)