Answer:
a)n=0.5 mol
b)n=0.5 mol
c)U/Q=0.714
Step-by-step explanation:
Given that
Mass of oxygen m= 16 g
Heated from 26.4°C to 111°C
Molar weight M=32 g/mol
a)
Number of mole,n
n=m/M
n=16/32 mol
n=0.5 mol
b)
Heat,Q
Q= n Cp ΔT
As we know that it is diatomic gas so
Cp=(7/2)R
Q=7 R n ΔT/2
Now by putting the values
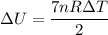 ----1
----1
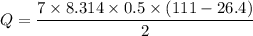
Q=1230.88 J
We know that internal energy
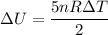 --------2
--------2
From equation 1 and 2
U/Q= 5/7
U/Q=0.714