Answer:
The unknown base should be KOH whose molar mass is 56 g per mol (Answer)
Step-by-step explanation:
Molarity = moles /(Volume in liters)
So,
Moles = Molarity
 Volume
Volume
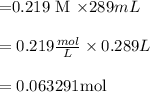
Molar mass = mass/moles
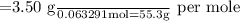
The unknown base should be KOH whose molar mass is 56 g per mol (Answer)