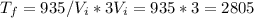Answer:
2805 °C
Step-by-step explanation:
If the gas in the tank behaves as ideal gas at the start and end of the process. We can use the following equation:
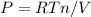
The key issue is identify the quantities (P,T, V, n) in the initial and final state, particularly the quantities that change.
In the initial situation the gas have an initial volume
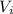 , temperature
, temperature
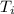 , and pressure
, and pressure
 ,.
,.
And in the final situation the gas have different volume
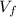 and temeperature
and temeperature
 , the same pressure
, the same pressure
 ,, and the same number of moles
,, and the same number of moles
 ,.
,.
We can write the gas ideal equation for each state:
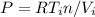 and
and
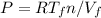 , as the pressure are equals in both states we can write
, as the pressure are equals in both states we can write
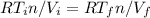
solving for

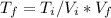 (*)
(*)
We know
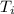 = 935 °C, and that the
= 935 °C, and that the
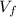 (the complete volume of the tank) is the initial volume
(the complete volume of the tank) is the initial volume
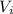 plus the part initially without gas which has a volume twice the size of the initial volume (read in the statement: the other side has a volume twice the size of the part containing the gas). So the final volume
plus the part initially without gas which has a volume twice the size of the initial volume (read in the statement: the other side has a volume twice the size of the part containing the gas). So the final volume
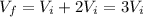
Replacing in (*)