Answer:
the maximum volume that can be burned per minute is: 0,895 mL of ethanol.
Step-by-step explanation:
The combustion of ethanol is:
C₂H₅OH + 3 O₂ → 2 CO₂ + 3 H₂O
With gas law:
PV/RT = n
Where P is pressure (1,00 atm)
V is volume (4,73 L of air per minute)
R is gas constant (0,082 atmL/molK)
T is temperatue(25°C≡298,15K)
And n are moles, replacing:
n = 0,193 moles of air per minute.
These moles of air contain:
0,193 moles air ×
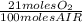 = 0,0406 moles O₂
= 0,0406 moles O₂
Thus, the maximum volume that can be burned per minute is:
0,046 moles O₂
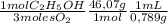 = 0,895 mL of ethanol per minute
= 0,895 mL of ethanol per minute
I hope it helps!