Answer:
Step-by-step explanation:
Rutherford gold experiment gave the explanation to some observations made on atoms.
When he shot the gold foil, most of the
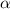 -particles passes through the foil undeflected nor absorbed. This faction of the particles propagated the foil as if there was nothing placed on their path.
-particles passes through the foil undeflected nor absorbed. This faction of the particles propagated the foil as if there was nothing placed on their path.
The surprising behavior of a few of the
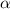 -particles was that they were deflected from their initial path on hitting the gold foil.
-particles was that they were deflected from their initial path on hitting the gold foil.
This implies that there is a part of the gold foil which has the same charge as the
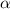 -particles, and more massive than the particles. Thus, he was able to determine the nucleus of an atom.
-particles, and more massive than the particles. Thus, he was able to determine the nucleus of an atom.