Answers and explanation:
The factor label method, also known as dimentional analysis, is a way to do conversions.
To do it you use fractions with units, for example this is how you would do question one:
20 mL ×
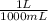 = 0.02 L
= 0.02 L
Since 1L = 1000mL, we put the fraction
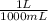 . The litres are on top because they are the unit we want, while the mL are on the bottom so that they will cancel out. Certain questions may require more than one step of this.
. The litres are on top because they are the unit we want, while the mL are on the bottom so that they will cancel out. Certain questions may require more than one step of this.
We can use this same method with all the other questions:
2. 950 g ×
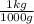 = 0.95 kg
= 0.95 kg
3. 55 mm ×
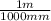 = 0.055 m
= 0.055 m
4. 200 in ×
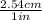 = 508 cm
= 508 cm
5. 25 cm ×
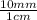 = 250 mm
= 250 mm
6. 0.005 kg ×
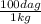 = 0.5 dag
= 0.5 dag
7. 2 hrs ×
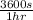 = 7200 s
= 7200 s
8. 15 g ×
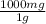 = 15000 mg
= 15000 mg
9. 1.3 yrs ×
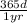 ×
×
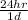 = 11388 hrs
= 11388 hrs
10. 0.075 m ×
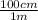 = 7.5 cm
= 7.5 cm