Answer:
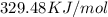 heat is released by the combustion of
heat is released by the combustion of
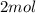 of methane
of methane
Step-by-step explanation:
The value of enthalpy determines whether the reaction is exothermic or endothermic. If the enthalpy change is positive, then the reaction is endothermic (heat or energy released) and if the enthalpy change is negative then the reaction is exothermic (heat or energy absorbed).
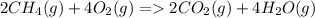
![\Delta H \ reaction=[ 2(\Delta HfCO_2)+ 4(\Delta HfH_2O)]-[2(\Delta HfCH_4)+4(\Delta HfO_2)]](https://img.qammunity.org/2020/formulas/biology/middle-school/u5j07j5au4m5p5qxjnwrppibp1jiyoxm9g.png)
=
![2 ( -(393.5 KJ)/mol)-[2( -74.6 KJ/mol)+4(-241.82 KJ/mol)]](https://img.qammunity.org/2020/formulas/biology/middle-school/edn7afixn3vwrxunp2dxpvjohpethuvp3c.png)
![= -787 KJ/mol-[ -149.2 KJ/mol-967.28 KJ/mol]](https://img.qammunity.org/2020/formulas/biology/middle-school/bf9autlq42gzpdaybic6gsa9gewztm8xkb.png)
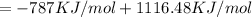
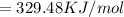
In this question, the enthalpy of formation has positive value and hence the reaction is endothermic in which the heat is released.