Answer:
The temperature of well water be 288.07 K
Step-by-step explanation:
We have given Heat in house
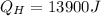
Work done W = 900 J
Efficiency is given by
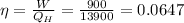
Efficiency is also given by
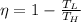 , here
, here
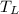 is lower temperature and
is lower temperature and
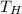 is higher temperature
is higher temperature
So
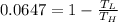
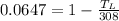
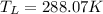
So the temperature of well water be 288.07 K