Answer:
Number of electrons, n = 6
Step-by-step explanation:
Total charge in a single droplet,
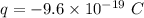
The measured charge of any single droplet,
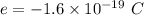
Let n is the number of excess electrons are contained within the drop. According to the quantization of charge :
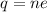
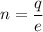
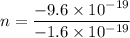
n = 6
So, there are 6 electrons contained within the drop. Hence, this is the required solution.