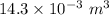Answer:
The change of the volume of the device during this cooling is
Step-by-step explanation:
Given that,
Mass of oxygen = 10 g
Pressure = 20 kPa
Initial temperature = 110°C
Final temperature = 0°C
We need to calculate the change of the volume of the device during this cooling
Using formula of change volume
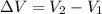
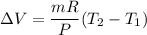
Put the value into the formula
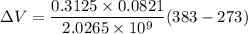
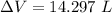
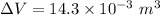
Hence, The change of the volume of the device during this cooling is