Answer:
The concentration of the standard NaOH solution is 0.094 moles/L.
Step-by-step explanation:
In the titration, the equivalence point is defined as the point where the moles of NaOH (the titrant) and KHP (the analyte) are equal:
moles of NaOH = moles of KHP
![[NaOH]xV_(NaOH) = moles of KHP](https://img.qammunity.org/2020/formulas/chemistry/high-school/znamf962ks3hl20szfd6ajin2jd0xzmw0k.png)
![[NaOH] = (moles of KHP)/(V_(NaOH))](https://img.qammunity.org/2020/formulas/chemistry/high-school/l74umbc7upvwmu2274vvijp22bn4rn9o0p.png)
The
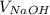 is 40.82mL = 0.04082L and the moles of KHP are
is 40.82mL = 0.04082L and the moles of KHP are
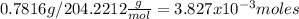
Replacing at the first equation:
![[NaOH] = (3.827x10^(-3)moles)/(0.04082L) = 0.094 moles/L](https://img.qammunity.org/2020/formulas/chemistry/high-school/ksy1bbjya6hnvfhwvkw72li3n6yx6ve89c.png)