Answer:
C₁₀H₂₀O
Step-by-step explanation:
The molecular formula must be
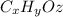 . The combustion reaction will occur between the fuel and oxygen gas:
. The combustion reaction will occur between the fuel and oxygen gas:
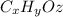 + O₂ → CO₂ + H₂O
+ O₂ → CO₂ + H₂O
For Lavoisier law, the mass of the reactants must be equal to the mass of the products (mass conservation):
10.0 + mO₂ = 28.16 + 11.53
mO₂ = 29.69 mg
Supposing that all the oxygen and the menthol were consumed, let's calculate the number of moles of the compounds, knowing, for the Periodic Table, that:
MC = 12 g/mol, MO = 16 g/mol, MH = 1 g/mol
MCO₂ = 12 + 2x16 = 44 g/mol
MH₂O = 2x1 + 16 = 18 g/mol
MO₂ = 2x16 = 32 g/mol
n = mass (g)/molar mass
nCO₂ = 0.02816/44 = 6.4x10⁻⁴ mol
nH₂O = 0.01153/18 = 6.4x10⁻⁴ mol
nO₂ = 0.02969/32 = 9.3x10⁻⁴ mol
The molar number is proportional in the molecule, so, in CO₂, the number of C is 6.4x10⁻⁴ mol, and of O is 1.28x10⁻³. All the carbon of the methol will be in CO₂, and all the H will be in H₂O. The number of moles of O will be the difference of moles in H₂O and the O₂, then:
nC = 6.4x10⁻⁴ mol
nH = 2x6.4x10⁻⁴ = 1.28x10⁻³ mol
nO = (2x6.4x10⁻⁴ + 6.4x10⁻⁴) - (2x9.3x10⁻⁴) = 6.0x10⁻⁵
The empirical formula is the molecule formula with the small subscripts numbers, which represent the number of moles of the atoms in the molecule. So, let's divide all the number of moles for the small on 6.0x10⁻⁵.
nC = (6.4x10⁻⁴)/(6.0x10⁻⁵) = 10
nH = (1.28x10⁻³)/(6.0x10⁻⁵) = 20
nO = (6.0x10⁻⁵)/(6.0x10⁻⁵) = 1
So, the empirical formula of methol is C₁₀H₂₀O.