Answer:
72.6 atm should be the pressure at which acetylene tank.
Step-by-step explanation:
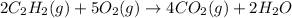
Let the temperature of the both tanks be same as T.
Volume of the tank in which oxygen is filled =
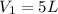
Pressure of the oxygen in tank =
 = 127 atm
= 127 atm
According to reaction 5 moles of oxygen reacts with 2 moles of acetylene.
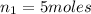
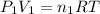
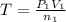 ..[1]
..[1]
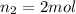
Volume of the tank in which acetylene is filled =
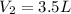
Pressure of the acetylene in tank =
 = ?
= ?
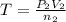 ..[2]
..[2]
[1] = [2]
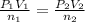
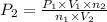
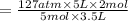
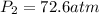
72.6 atm should be the pressure at which acetylene tank.