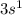Answer:
electron configuration:
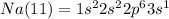
atomic orbital with the highest energy value
Step-by-step explanation:
The electrons are spinning around the nucleus in different orbitals. When we get the electron configuration, we see how these electrons are organized around the nucleus. Every orbital has a different energy value and the electrons that are in the same orbital have the same energy value. When an orbital is closer to the nucleus have a lower energy value, so those electrons that are furthest from the nucleus have higher levels of energy and are the ones that participate in chemical bonds these electrons are also called valence electrons.
The electronic orbitals are organized in 7 levels and each level has sub-levels, four in total, these are s,p,d,f. Level 1 is the closest to the nucleus so it has the lowest energy value. In addition, not all the levels have four sub-levels.
Each sub-level can have just certain number of electrons (s=2, p=6, d=10, f=14) and each level can have just certain number of sub-levels
level 1 = 1 sub-level (s)
level 2 = 2 sub-levels (s,p)
level 3 =3 sub-levels (s,p,d)
level 4 = 4 sub-levels (s,p,d,f)
level 5 = 4 sub-levels (s,p,d,f)
level 6 = 3 sub-levels (s,p,d)
level 7 = 2 sub-levels (s,p)
To organize the electrons in around the nucleus we must start with the level that has the lower energy, level 1 and so on.
The sodium Na has 11 electrons
We start organizing them in the lowest level that is 1, this have just one sub-level, s, and this sub-level can have just 2 electrons.
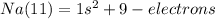
We have 9 electrons to organize. Now we use the level 2 that has 2 orbitals s=2 electrons and p=6 electrons. 8 electrons in total.
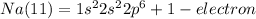
We still have 1 electron. For this electron we go to the next level, 3, using the orbital is enough for this electron. So, the electronic configuration for Na (11) is:
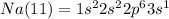
We said before that the closest to the nucleus the lower the energy. So the electron with the most energy will be in the higher level that for Na(11) is